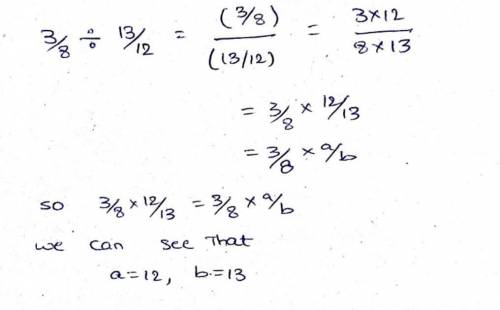Chemistry, 14.01.2020 22:31 naenae2cold12021
Consider the following equivalent expressions: 38÷1312 and 38? ab what are the values of a and b? enter the value of a followed by the value of b, separated by a comma.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
Consider the following equivalent expressions: 38÷1312 and 38? ab what are the values of a and b?...
Questions

Mathematics, 11.12.2021 03:10


SAT, 11.12.2021 03:10

Mathematics, 11.12.2021 03:10

Biology, 11.12.2021 03:10

Mathematics, 11.12.2021 03:10

English, 11.12.2021 03:10

English, 11.12.2021 03:10

Physics, 11.12.2021 03:10

Mathematics, 11.12.2021 03:10

Mathematics, 11.12.2021 03:10



Mathematics, 11.12.2021 03:10





Mathematics, 11.12.2021 03:10

History, 11.12.2021 03:10