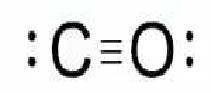Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3


Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1
You know the right answer?
Use formal charge arguments to explain why co has a much smaller dipole moment than would be expecte...
Questions

History, 04.11.2020 22:10

English, 04.11.2020 22:10



Advanced Placement (AP), 04.11.2020 22:10

Mathematics, 04.11.2020 22:10

History, 04.11.2020 22:10

Mathematics, 04.11.2020 22:10




Mathematics, 04.11.2020 22:10

Mathematics, 04.11.2020 22:10

History, 04.11.2020 22:10

Mathematics, 04.11.2020 22:10

Mathematics, 04.11.2020 22:10

History, 04.11.2020 22:10

English, 04.11.2020 22:10

Spanish, 04.11.2020 22:10

Mathematics, 04.11.2020 22:10