Chemistry, 14.01.2020 23:31 lauraabosi
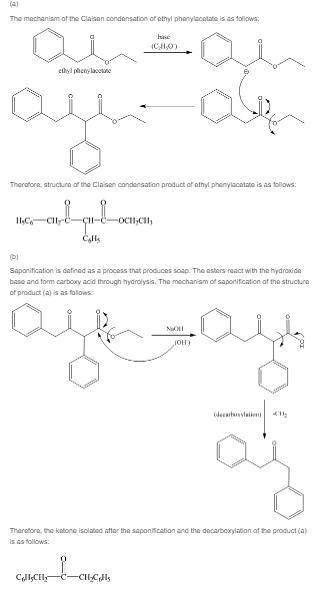
(a) give the structure of the claisen condensation product of ethyl phenylacetate
(c6h5ch2cooch2ch3).
(b) what ketone would you isolate after saponification and decarboxylation of this claisen condensation product?
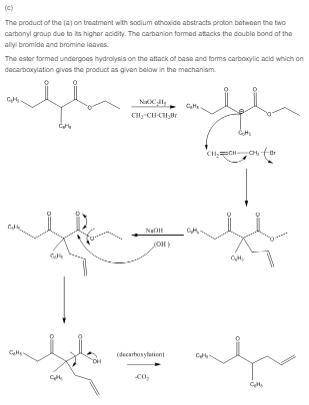
(c) what ketone would you isolate after treatment of the claisen condensation product of ethyl phenylacetate with sodium ethoxide and allyl bromide, followed by saponification and decarboxylation?
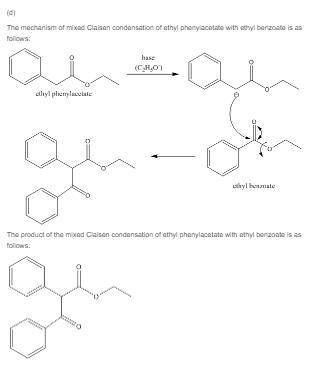
(d) give the structure of the mixed claisen condensation product of ethyl phenylacetate and ethyl benzoate.
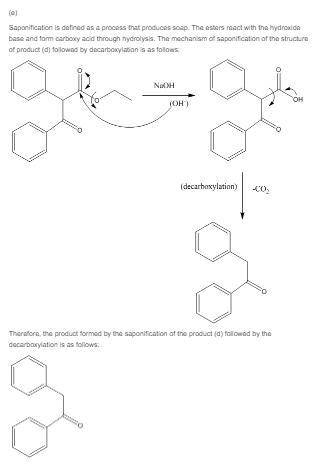
(e) what ketone would you isolate after saponification and decarboxylation of the product in part (d)?
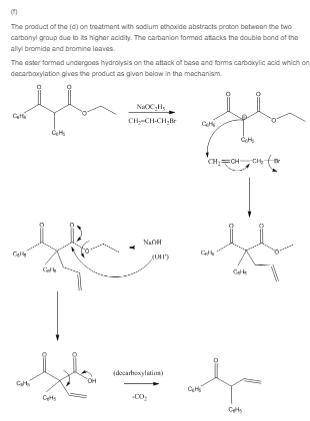
(f) what ketone would you isolate after treatment of the product in part (d) with sodium ethoxide and allyl bromide, followed by saponification and decarboxylation?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3
You know the right answer?
(a) give the structure of the claisen condensation product of ethyl phenylacetate
(c6h5ch2coo...
(c6h5ch2coo...
Questions




English, 07.05.2020 14:57

Mathematics, 07.05.2020 14:57







History, 07.05.2020 14:57

Mathematics, 07.05.2020 14:57

Mathematics, 07.05.2020 14:57