Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:20
Both 1,2−dihydronaphthalene and 1,4−dihydronaphthalene may be selectively hydrogenated to 1,2,3,4−tetrahydronaphthalene. one of these isomers has a heat of hydrogenation of 101 kj/mol (24.1 kcal/mol), and the heat of hydrogenation of the other is 113 kj/mol (27.1 kcal/mol). match the heat of hydrogenation with the appropriate dihydronaphthalene.
Answers: 2

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3

Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2

Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 1
You know the right answer?
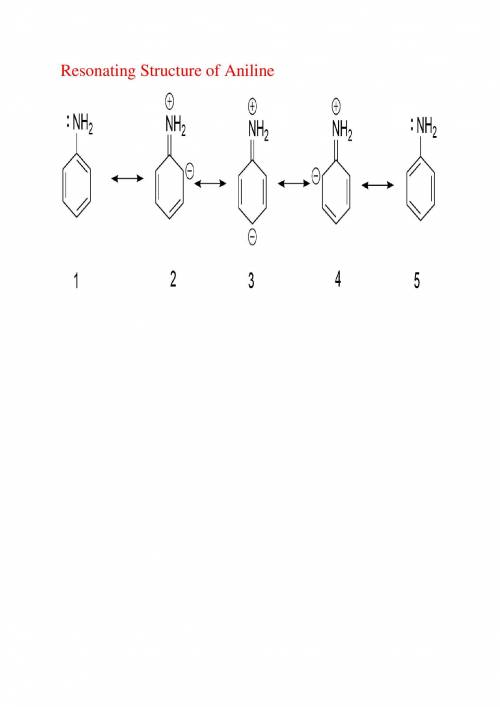
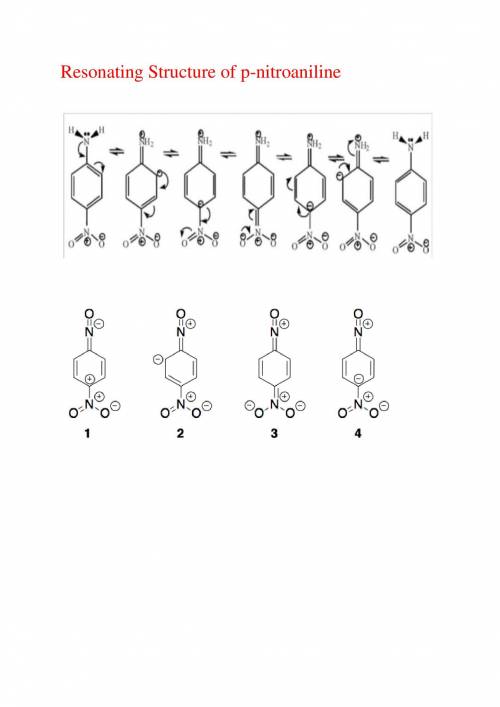
As the extent of electron delocalization into the ring increases, the geometry at nitrogen flattens....
Questions

Biology, 01.09.2020 19:01

History, 01.09.2020 19:01


Mathematics, 01.09.2020 19:01


Mathematics, 01.09.2020 19:01

English, 01.09.2020 19:01

Mathematics, 01.09.2020 19:01

Computers and Technology, 01.09.2020 19:01



Social Studies, 01.09.2020 19:01


Mathematics, 01.09.2020 19:01


Engineering, 01.09.2020 19:01

Biology, 01.09.2020 19:01



Mathematics, 01.09.2020 19:01