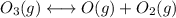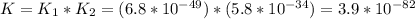Consider the following data. no2(g) equilibrium reaction arrow identifying the requirement for light no(g) + o(g) k = 6.8 ✕ 10-49 o3(g) + no(g) equilibrium reaction arrow no2(g) + o2(g) k = 5.8 ✕ 10-34 calculate a value for the equilibrium constant for the reaction below. (hint: when reactions are added together, the equilibrium expressions are multiplied.)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
Consider the following data. no2(g) equilibrium reaction arrow identifying the requirement for light...
Questions


Mathematics, 31.01.2022 19:10

Spanish, 31.01.2022 19:10

Mathematics, 31.01.2022 19:10


Computers and Technology, 31.01.2022 19:10

Mathematics, 31.01.2022 19:10

Mathematics, 31.01.2022 19:10


Chemistry, 31.01.2022 19:10

Spanish, 31.01.2022 19:10



Mathematics, 31.01.2022 19:20

Health, 31.01.2022 19:20

English, 31.01.2022 19:20



Mathematics, 31.01.2022 19:20

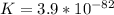
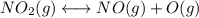
![K_1=\frac{[NO][O]}{[NO_2]}=6.8*10^{-49}](/tpl/images/0455/4423/c0bdd.png)

![K_2=\frac{[NO_2}{[O_3][NO]}=5.8*10^{-34}](/tpl/images/0455/4423/ae794.png)