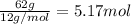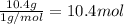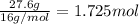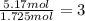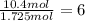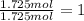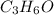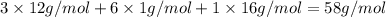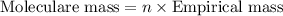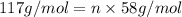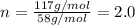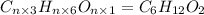Chemistry, 15.01.2020 04:31 alondrasanchezvillan
Compound x, isolated from lanolin (sheep's wool fat), has the pungent aroma of dirty sweatsocks. a careful analysis showed that compound x contains 62% carbon and 10.4% hydrogen. no nitrogen or halogen was found.
a. compute an empirical formula for compound x.
express your answer as a condensed structural formula.
b. a molecular weight determination showed that compound x has a molecular weight of approximately 117. find the molecular formula of compound x.
express your answer as a condensed structural formula.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1


Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
Compound x, isolated from lanolin (sheep's wool fat), has the pungent aroma of dirty sweatsocks. a c...
Questions


Mathematics, 03.05.2020 14:22

History, 03.05.2020 14:22

English, 03.05.2020 14:22

English, 03.05.2020 14:22


Biology, 03.05.2020 14:22

Mathematics, 03.05.2020 14:22



Chemistry, 03.05.2020 14:22

Social Studies, 03.05.2020 14:22

Engineering, 03.05.2020 14:22

Mathematics, 03.05.2020 14:22

Biology, 03.05.2020 14:23




Mathematics, 03.05.2020 14:23

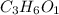 .
.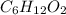 .
.