Chemistry, 15.01.2020 05:31 babygreg2001p97abr
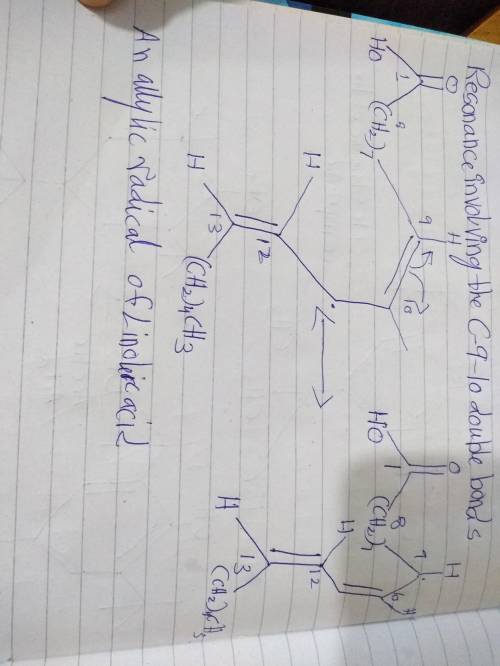
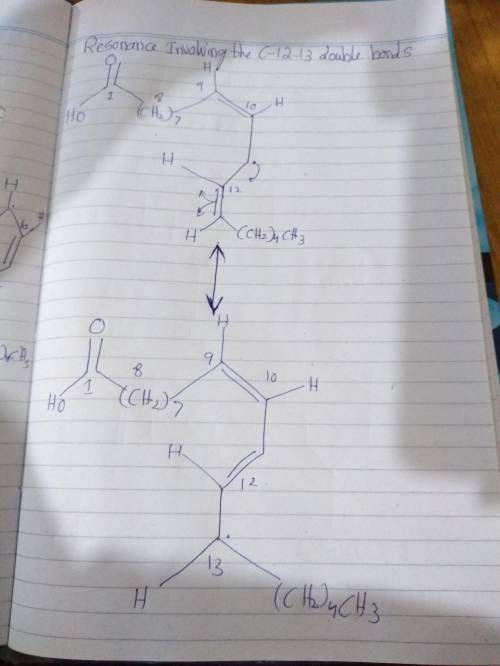
The allyl radical of linoleic acid is stabilized by resonance involving the c-9-10 and c-12-13 double bonds. show structures for each of these resonance contributors.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 22.06.2019 00:30
13. calculate the initial concentration (before precipitation) of carbonate ions after the addition of each 0.05 ml of solution b to the 1.00 l beaker of solution a. divide the work among group members and write the answers in the table in model 3. assume the volume change as solution b is added is negligible. 14. notice the initial concentrations of zn2+ - and cu2+ in the table in model 3. a. explain how these were obtained from the data in model 2. b. as solution b is added and precipitates form, do these initial concentrations change? 15. use the data in model 2 to indicate the presence of precipitate (either znco3 or cuco3) after each 0.05 ml addition of solution b in model 3. 16. use the initial concentrations of carbonate ions and zinc ions to calculate the reaction quotient, qsp for the zinc carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3. 17. use the initial concentrations of carbonate ion and copper(ii) ions to calculate the qsp for the copper(ii) carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3.
Answers: 3

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

You know the right answer?
The allyl radical of linoleic acid is stabilized by resonance involving the c-9-10 and c-12-13 doubl...
Questions





History, 21.08.2019 10:00

Physics, 21.08.2019 10:00

Biology, 21.08.2019 10:00


History, 21.08.2019 10:00


Mathematics, 21.08.2019 10:00



Mathematics, 21.08.2019 10:00

English, 21.08.2019 10:00