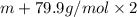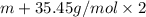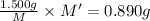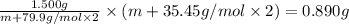Chemistry, 15.01.2020 19:31 whitakers87
An element x has a dibrom ide with the empirical formula xbr2 and a dichloride with the empirical formula xci2. the dibromide is completely converted to the dichloride when it is heated in a stream of chlorine according to the reaction $$xbr2 + cl2 → xci2 + br2$$ when 1.500 g xbr2 is treated, 0.890 g xci2 results. (a) calculate the atomic mass of the element x. (b) by reference to a list of the atomic masses of the elements, identify the element x.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which substance absorbs 58.16 kj of energy when 3.11 mol vaporizes? a)ch4 b)h2s c)co2 d)nacl
Answers: 2

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
An element x has a dibrom ide with the empirical formula xbr2 and a dichloride with the empirical fo...
Questions


English, 01.06.2021 19:00


Mathematics, 01.06.2021 19:00










Chemistry, 01.06.2021 19:00






Biology, 01.06.2021 19:00

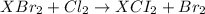
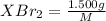 mol
mol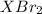 gives 1 mole of
gives 1 mole of 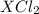 . then
. then 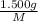 mol of [tex[CBr_2[/tex] will give:
mol of [tex[CBr_2[/tex] will give: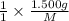 mol=\frac{1.500 g}{M}[/tex] mol[/tex] of
mol=\frac{1.500 g}{M}[/tex] mol[/tex] of