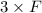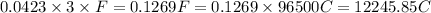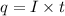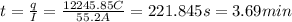Chemistry, 15.01.2020 20:31 squawk1738
How many minutes will it take to plate out 2.19 g of chromium metal from a solution of cr3+cr3+ using a current of 55.2 amps in an electrolyte cell?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
How many minutes will it take to plate out 2.19 g of chromium metal from a solution of cr3+cr3+ usin...
Questions

Mathematics, 06.03.2021 22:50


Medicine, 06.03.2021 22:50


Health, 06.03.2021 22:50



Mathematics, 06.03.2021 22:50


Mathematics, 06.03.2021 23:00

Mathematics, 06.03.2021 23:00



Mathematics, 06.03.2021 23:00

Mathematics, 06.03.2021 23:00

English, 06.03.2021 23:00





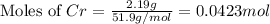

 =
=