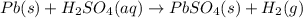The equation shows lead reacting with sulfuric acid to produce lead sulfate and hydrogen gas.
upper p b (s) plus upper h subscript 2 upper s upper o subscript 4 (a q) right arrow upper p b upper s upper o subscript 4 (s) plus upper h subscript 2 (g).
which type of chemical reaction does this equation represent?
synthesis
decomposition
single replacement
double replacement

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
The equation shows lead reacting with sulfuric acid to produce lead sulfate and hydrogen gas.
<...
<...
Questions


Chemistry, 03.06.2020 13:09

Biology, 03.06.2020 13:09



English, 03.06.2020 13:09


Mathematics, 03.06.2020 13:09




History, 03.06.2020 13:09


Mathematics, 03.06.2020 13:09


Mathematics, 03.06.2020 13:09



Mathematics, 03.06.2020 13:09

Mathematics, 03.06.2020 13:09