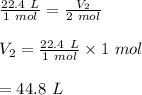Chemistry, 16.01.2020 00:31 bvolleyball9
What is the volume of 2 moles of methane (ch4)? (one mole of any gas occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this question.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2

Chemistry, 23.06.2019 09:30
The mass of a proton is approximately equal to the mass of
Answers: 1
You know the right answer?
What is the volume of 2 moles of methane (ch4)? (one mole of any gas occupies 22.4 l under certain...
Questions


Social Studies, 04.02.2020 11:43

Mathematics, 04.02.2020 11:43



History, 04.02.2020 11:43

Chemistry, 04.02.2020 11:43






English, 04.02.2020 11:43

Biology, 04.02.2020 11:43

English, 04.02.2020 11:43



Mathematics, 04.02.2020 11:43

English, 04.02.2020 11:43

Mathematics, 04.02.2020 11:43

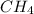 is 44.8 L.
is 44.8 L.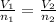 ......(1)
......(1)
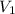 = the volume of 1 mol of
= the volume of 1 mol of 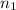 = number of moles
= number of moles 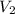 = the volume of 2.0 mol
= the volume of 2.0 mol 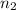 = the number of moles
= the number of moles