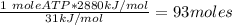Chemistry, 16.01.2020 05:31 JayLiz1737
Ask your teacher referring to the metabolic process below, calculate the maximum number of moles of atp that can be synthesized from adp from the breakdown of one mole of glucose. c6h12o6(s) + 6 o2(g) → 6 co2(g) + 6 h2o(l) δg° = −2880 kj/mol adp + h3po4 → atp + h2o δg° = 31 kj/mol webassign will check your answer for the correct number of significant figures. moles

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1


Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3
You know the right answer?
Ask your teacher referring to the metabolic process below, calculate the maximum number of moles of...
Questions



History, 26.06.2021 01:00


Business, 26.06.2021 01:00

Health, 26.06.2021 01:00



Mathematics, 26.06.2021 01:00

Chemistry, 26.06.2021 01:00


Mathematics, 26.06.2021 01:00

Computers and Technology, 26.06.2021 01:00

Social Studies, 26.06.2021 01:00

History, 26.06.2021 01:00


Biology, 26.06.2021 01:00

Mathematics, 26.06.2021 01:00


Mathematics, 26.06.2021 01:00

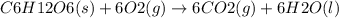 ΔG°=-2880 KJ/mol
ΔG°=-2880 KJ/mol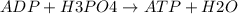 ΔG°=31 KJ/mol
ΔG°=31 KJ/mol