
Chemistry, 16.01.2020 07:31 tommyaberman
The gas-phase decomposition of ch3cho (g) occurs according to the equation
ch3cho (g) > ch4 (g) +co (g) and is second order. the value of the rate constant is 0.105 m-1 x s-1 at 490 degrees celcius. if the concentration of ch3cho (g) is 0.012 m initially, what will be its concentration 5.0 minutes later.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2
You know the right answer?
The gas-phase decomposition of ch3cho (g) occurs according to the equation
ch3cho (g) > ch...
ch3cho (g) > ch...
Questions



Social Studies, 21.10.2020 20:01


Mathematics, 21.10.2020 20:01

English, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01

Engineering, 21.10.2020 20:01

Computers and Technology, 21.10.2020 20:01


History, 21.10.2020 20:01

History, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01




Engineering, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01

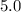 minutes would be
minutes would be 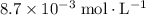 .
.![\text{Rate} = k \cdot [{\rm CH_3CHO\; (g)}]^{n}](/tpl/images/0457/4625/6c0ce.png) ,
, 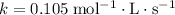 is the rate constant, and
is the rate constant, and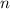 is the order of the reactant
is the order of the reactant ![[{\rm CH_3CHO\; (g)}]](/tpl/images/0457/4625/e1c1b.png) in this reaction.
in this reaction. 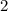 . By definition, the order of a reaction is the sum of the orders of all the reactants in the rate law. In this case, since
. By definition, the order of a reaction is the sum of the orders of all the reactants in the rate law. In this case, since ![[\rm CH_3CHO\; (g)]](/tpl/images/0457/4625/851e9.png) is the only reactant, its should must also be
is the only reactant, its should must also be 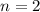 and
and ![\text{Rate} = k \cdot [{\rm CH_3CHO\; (g)}]^2](/tpl/images/0457/4625/e8149.png) .
. (with the unit
(with the unit 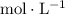 ) represent the concentration of
) represent the concentration of ![x = [\rm CH_3CHO\; (g)]](/tpl/images/0457/4625/7c9b5.png) .
.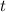 (in seconds.) (There should be a negative sign, since the concentration of the reactants was supposed to decrease.) In this case,
(in seconds.) (There should be a negative sign, since the concentration of the reactants was supposed to decrease.) In this case, ![\displaystyle \text{Rate} = -\frac{d}{dt}\, \left([\rm CH_3CHO\; (g)]\right) = -\frac{dx}{dt}](/tpl/images/0457/4625/bcf35.png) .
.![k\cdot [{\rm CH_3CHO\; (g)}]^2 = k \, x^2](/tpl/images/0457/4625/a921f.png) .
.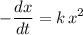 .
.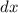 while
while 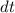 . Note that in this case there's no such variable as
. Note that in this case there's no such variable as 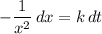 .
.![\displaystyle \int\left[-\frac{1}{x^2}\, dx\right] = \int\left[k\, dt\right]](/tpl/images/0457/4625/2fb86.png) .
.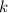 is simply a constant and is not dependent on the value of
is simply a constant and is not dependent on the value of 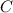 to one side of the equation.
to one side of the equation.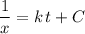 .
.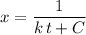 .
.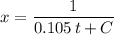 .
.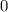 (initial condition,) the concentration is
(initial condition,) the concentration is 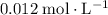 . Hence, the constant
. Hence, the constant 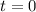 ,
, 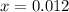 . Solve the equation for
. Solve the equation for 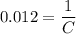 .
.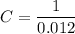 .
.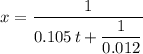 .
.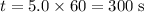 .
.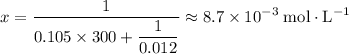 .
.

