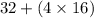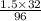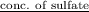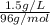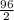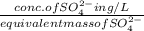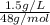Chemistry, 16.01.2020 18:31 jacksoneaton
An analytical chemist determines that an estuarine water sample contains 1.5 g/liter of sulfate ion (so4 2). what is the concentration in terms of: (a) g/l of sulfur? (b) molar concentration of sulfate? (c) normality?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2


Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
You know the right answer?
An analytical chemist determines that an estuarine water sample contains 1.5 g/liter of sulfate ion...
Questions






Mathematics, 11.02.2020 01:20







Mathematics, 11.02.2020 01:20


Mathematics, 11.02.2020 01:20




Mathematics, 11.02.2020 01:20

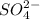 is as follows.
is as follows.