
Suppose we have a 12.2 l sample containing 0.50 mol oxygen gas at a pressure of 1 atm and a temperature of 25 degrees celcius. if all this oxygen were converted to ozone at the same temperature and pressure, what would be the volume of the ozone? balanced equation: 3o2 → 2o3a. 6.5lb. 2.8lc. 8.1ld. 7.4l

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Suppose we have a 12.2 l sample containing 0.50 mol oxygen gas at a pressure of 1 atm and a temperat...
Questions

Mathematics, 28.09.2019 05:20


Physics, 28.09.2019 05:20


Mathematics, 28.09.2019 05:20

History, 28.09.2019 05:20




History, 28.09.2019 05:20


Mathematics, 28.09.2019 05:20

English, 28.09.2019 05:20


History, 28.09.2019 05:20

Mathematics, 28.09.2019 05:20

Spanish, 28.09.2019 05:20

Social Studies, 28.09.2019 05:20


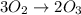
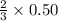 moles of ozone
moles of ozone

