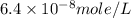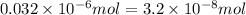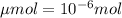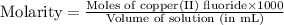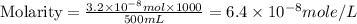Chemistry, 16.01.2020 20:31 starfox5454
Achemist prepares a solution of copper(ii) fluoride (cuf2) by measuring out 0.032 µmol of copper(ii) fluoride into a 500 ml volumetric flask and filling the flask to the mark with water. calculate the concentration in mol/l of the chemist's copper(ii) fluoride solution.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1
You know the right answer?
Achemist prepares a solution of copper(ii) fluoride (cuf2) by measuring out 0.032 µmol of copper(ii)...
Questions







Mathematics, 22.04.2020 01:46


Mathematics, 22.04.2020 01:46


Health, 22.04.2020 01:46




Mathematics, 22.04.2020 01:46

Chemistry, 22.04.2020 01:46


English, 22.04.2020 01:46