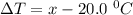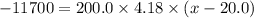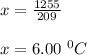Chemistry, 16.01.2020 21:31 mjakabeast24
Aglass containing 200.0 g of h2o at 20.0°c was placed in a refrigerator. the water loses 11.7 kj as it cools to a constant temperature. what is its new temperature? the specific heat of water is 4.184 j/g·°c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
Aglass containing 200.0 g of h2o at 20.0°c was placed in a refrigerator. the water loses 11.7 kj as...
Questions

Mathematics, 08.09.2020 09:01

Mathematics, 08.09.2020 09:01

Mathematics, 08.09.2020 09:01

Mathematics, 08.09.2020 09:01



Computers and Technology, 08.09.2020 09:01

Mathematics, 08.09.2020 09:01

Mathematics, 08.09.2020 09:01

Mathematics, 08.09.2020 09:01



Biology, 08.09.2020 09:01

Physics, 08.09.2020 09:01

Social Studies, 08.09.2020 09:01

Mathematics, 08.09.2020 09:01

Mathematics, 08.09.2020 09:01


History, 08.09.2020 09:01

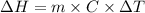
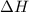 is the enthalpy change
is the enthalpy change
 is the temperature change
is the temperature change