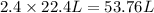Chemistry, 16.01.2020 22:31 BreBreDoeCCx
When calcium carbonate is added to hydrochloric acid, calcium chloride, carbon dioxide, and water are produced according to the balanced chemical equation below:
caco3(s) + 2hc1(aq) 4 cac12(aq) +h20(1) + co2(g)
imagine mixing 2.5 moles of calcium carbonate with 4.8 moles of hydrochloric acid. calculate the number of moles of caco3 and hcl used in the intro activity. determine the limiting reactant and use the ideal gas law to estimate the max volume of co2

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Now consider the reaction when 45.0 g naoh have been added. what amount of naoh is this, and what amount of fecl3 can be consumed by it?
Answers: 3

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1
You know the right answer?
When calcium carbonate is added to hydrochloric acid, calcium chloride, carbon dioxide, and water ar...
Questions


English, 22.07.2020 01:01


Mathematics, 22.07.2020 01:01











Computers and Technology, 22.07.2020 01:01





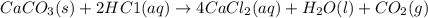
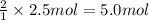 of HCl
of HCl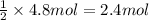 of calcium carbonate
of calcium carbonate