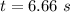Chemistry, 16.01.2020 23:31 jayjinks976
For the simple decomposition reactionab(g)→ a(g) + b(g)rate =k[ab]2 and k=0.2 l/mol*s . how long will it takefor [ab] to reach 1/3 of its initial concentration 1.50 mol/l?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1
You know the right answer?
For the simple decomposition reactionab(g)→ a(g) + b(g)rate =k[ab]2 and k=0.2 l/mol*s . how long wil...
Questions

Computers and Technology, 29.01.2020 23:00

History, 29.01.2020 23:00

Chemistry, 29.01.2020 23:00







Mathematics, 29.01.2020 23:01

Mathematics, 29.01.2020 23:01



Mathematics, 29.01.2020 23:01



Mathematics, 29.01.2020 23:01

Mathematics, 29.01.2020 23:01

Physics, 29.01.2020 23:01

![Rate = k[AB]^2](/tpl/images/0458/3241/7f1de.png)
![\frac{1}{[A_t]} = \frac{1}{[A]_0}+kt](/tpl/images/0458/3241/f2ee3.png)
![[A_0]](/tpl/images/0458/3241/9a686.png) is the initial concentration = 1.50 mol/L
is the initial concentration = 1.50 mol/L![[A_t]](/tpl/images/0458/3241/5262c.png) is the final concentration = 1/3 of initial concentration =
is the final concentration = 1/3 of initial concentration =  = 0.5 mol/L
= 0.5 mol/L