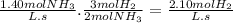Chemistry, 16.01.2020 23:31 kylabreanne120
If the average rate of appearance of nh3 in the following reaction is 1.40 m/s, what is the average rate of disappearance of h2 during the same time period?
n2(g) + 3h2(g) → 2nh3(g)
a. 5.40 m/s
b. 2.80 m/s
c. 1.20 m/s
d. 0.700 m/s
e. 2.10 m/s

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3

You know the right answer?
If the average rate of appearance of nh3 in the following reaction is 1.40 m/s, what is the average...
Questions

Computers and Technology, 25.06.2019 21:30




Mathematics, 25.06.2019 21:30

History, 25.06.2019 21:30


Mathematics, 25.06.2019 21:30




Social Studies, 25.06.2019 21:30

Mathematics, 25.06.2019 21:30







English, 25.06.2019 21:30