
Chemistry, 17.01.2020 00:31 pineapplepizaaaaa
Which of the following statements about standard reduction potential and spontaneity are true?
choose one or more:
a. a reaction with a negative gibbs standard free energy is thermodynamically spontaneous under standard conditions.
b. a coupled redox reaction with a negative standard reduction potential is thermodynamically spontaneous.
c. a coupled redox reaction with a positive standard reduction potential îeââ² is thermodynamically spontaneous.
d. a reaction with a positive gibbs standard free energy is thermodynamically spontaneous under standard conditions.

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
You know the right answer?
Which of the following statements about standard reduction potential and spontaneity are true?
Questions

History, 04.11.2019 18:31


Mathematics, 04.11.2019 18:31

Mathematics, 04.11.2019 18:31







Mathematics, 04.11.2019 18:31




Biology, 04.11.2019 18:31

Biology, 04.11.2019 18:31

Mathematics, 04.11.2019 18:31


History, 04.11.2019 18:31

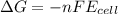 (1)
(1) 

