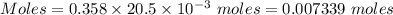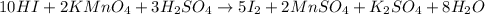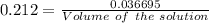Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
How many milliliters of a 0.212 m hi solution are needed to reduce 20.5 ml of a 0.358 m kmno4 soluti...
Questions

Geography, 27.11.2019 11:31



Social Studies, 27.11.2019 11:31



Social Studies, 27.11.2019 11:31


Mathematics, 27.11.2019 11:31


English, 27.11.2019 11:31



Mathematics, 27.11.2019 11:31

Mathematics, 27.11.2019 11:31

Mathematics, 27.11.2019 11:31

English, 27.11.2019 11:31

Mathematics, 27.11.2019 11:31

Biology, 27.11.2019 11:31

History, 27.11.2019 11:31

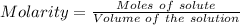
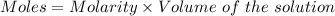
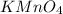 :
: