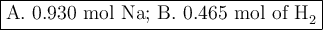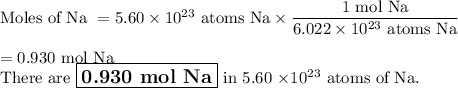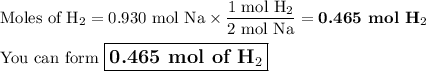Chemistry, 17.01.2020 07:31 luiscastaenda
How many moles are in 5.60 x 10^23 atoms of na? how many moles of h2 can be formed from this amount of na?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
How many moles are in 5.60 x 10^23 atoms of na? how many moles of h2 can be formed from this amount...
Questions


Biology, 10.04.2020 07:24

Mathematics, 10.04.2020 07:24

Biology, 10.04.2020 07:24

Mathematics, 10.04.2020 07:24

Mathematics, 10.04.2020 07:24

Chemistry, 10.04.2020 07:24


English, 10.04.2020 07:24

English, 10.04.2020 07:24



Medicine, 10.04.2020 07:24

Mathematics, 10.04.2020 07:24


Mathematics, 10.04.2020 07:24


Biology, 10.04.2020 07:25