
Based on the standard free energies of formation, which of the following reactions represent a feasible way to synthesize the product?
a. 2c(s)+h2(g)→c2h2(g); δg∘f=209.2 kj/mol
b. n2(g)+3h2(g)→2nh3(g); δg∘f=−33.30 kj/mol
c. 2c(s)+2h2(g)→c2h4(g); δg∘f=68.20 kj/mol
d. 2so(g)+o2(g)→2so2(g); δg∘f=−600.4 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1
You know the right answer?
Based on the standard free energies of formation, which of the following reactions represent a feasi...
Questions







Mathematics, 05.03.2020 07:20






English, 05.03.2020 07:20




Mathematics, 05.03.2020 07:21



Computers and Technology, 05.03.2020 07:21

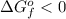 represents the a feasible way to synthesize the product. So, the correct answer are option B and D.
represents the a feasible way to synthesize the product. So, the correct answer are option B and D.

