
Chemistry, 18.01.2020 00:31 gabriella1232002
Raising the temperature of 10.0 g of water from 10.0°c to 20.0°c requires 100.0 cal of energy, while raising the temperature of 10.0 g of aluminum from 10.0°c to 20.0°c requires 22 cal. more calories are required to heat the water

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Raising the temperature of 10.0 g of water from 10.0°c to 20.0°c requires 100.0 cal of energy, while...
Questions




History, 20.04.2021 22:10


Mathematics, 20.04.2021 22:10


Mathematics, 20.04.2021 22:10



Chemistry, 20.04.2021 22:10

Chemistry, 20.04.2021 22:10


English, 20.04.2021 22:10



History, 20.04.2021 22:10



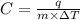
 = change in temperature of substance
= change in temperature of substance

