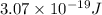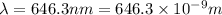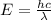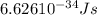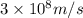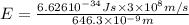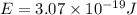When an electron in a 2p orbital of a particular atom makes a transition to the 2s orbital, a photon of approximate wavelength 646.3 nm is emitted. the energy difference between these 2p and 2s orbitals is: . 3.07 ã 10^â28 jb. 3.07 ã 10^â19 jc. 3.07 ã 10^â17 jd. 1.28 ã 10^â31 je. none of these

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1


Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1
You know the right answer?
When an electron in a 2p orbital of a particular atom makes a transition to the 2s orbital, a photon...
Questions

English, 28.12.2019 07:31

Mathematics, 28.12.2019 07:31

English, 28.12.2019 07:31



Mathematics, 28.12.2019 07:31

Mathematics, 28.12.2019 07:31

History, 28.12.2019 07:31



Mathematics, 28.12.2019 07:31

Mathematics, 28.12.2019 07:31



Mathematics, 28.12.2019 07:31



Chemistry, 28.12.2019 07:31

Mathematics, 28.12.2019 07:31

Mathematics, 28.12.2019 07:31