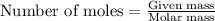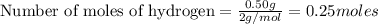Chemistry, 18.01.2020 01:31 josiebranstetter93
Two flexible containers for gases are at the same temperature and pressure. one holds 0.50 gram of hydrogen and the other holds 8.0 grams of oxygen. which of the following statements regarding these gas samples is false? the volume of the hydrogen container is the same as the volume of the oxygen container.
a the number of molecules in the hydrogen container is the same as the number of molecules in the oxygen container.
b the density of the hydrogen sample is less than that of the oxygen sample.
c the average kinetic energy of the hydrogen molecules is the same as the average kinetic energy of the oxygen molecules.
d the average speed of the hydrogen molecules is the same as the average speed of the oxygen molecules.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3


Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2
You know the right answer?
Two flexible containers for gases are at the same temperature and pressure. one holds 0.50 gram of...
Questions


Mathematics, 20.01.2021 21:20


Mathematics, 20.01.2021 21:20





Mathematics, 20.01.2021 21:20




Mathematics, 20.01.2021 21:20


Spanish, 20.01.2021 21:20

Mathematics, 20.01.2021 21:20

Mathematics, 20.01.2021 21:20

Computers and Technology, 20.01.2021 21:20


 of particles.
of particles.