

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2
You know the right answer?
Avoltaic cell consists of a hg/hg22+ electrode (e°= 0.85 v) and a sn/sn2+ electrode (e°= –0.14 v). c...
Questions



Biology, 03.08.2019 13:50

Mathematics, 03.08.2019 13:50

Mathematics, 03.08.2019 13:50

History, 03.08.2019 13:50


Spanish, 03.08.2019 13:50


Mathematics, 03.08.2019 13:50

History, 03.08.2019 13:50



Health, 03.08.2019 13:50

Mathematics, 03.08.2019 13:50

Mathematics, 03.08.2019 13:50


Mathematics, 03.08.2019 13:50


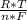 *ln(Q)
*ln(Q)![\frac{[C]^{c}*[D]^{d} }{[A]^{a}*[B]^{b} }](/tpl/images/0460/2641/2f4bf.png) In this case [C] and [D], they refer to the partial pressures, also known as molar concentrations in the case of gases or ions in solution, for the reaction products. [A] and [B] are also partial pressures but in the case of reagents. The exponents refer to the amount of moles that make up each substance that is participating in the reaction. Substances that are in a solid state have a unit concentration (that is, their value is 1), so they do not appear in Q.
In this case [C] and [D], they refer to the partial pressures, also known as molar concentrations in the case of gases or ions in solution, for the reaction products. [A] and [B] are also partial pressures but in the case of reagents. The exponents refer to the amount of moles that make up each substance that is participating in the reaction. Substances that are in a solid state have a unit concentration (that is, their value is 1), so they do not appear in Q.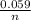 *ln(Q) Equation (A)
*ln(Q) Equation (A)![\frac{[Sn^{2+}] }{[Hg_{2} ^{+2} ]}](/tpl/images/0460/2641/af92b.png) where [Hg₂²⁺] is 0.24 M
where [Hg₂²⁺] is 0.24 M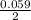 *ln
*ln![\frac{[Sn]^{2+} }{0.24}](/tpl/images/0460/2641/8f497.png)



