

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1

Chemistry, 23.06.2019 04:00
Silver reacts with oxygen to produce silver oxide. (write balanced chemical equation and identify type of chemical reaction.)
Answers: 1
You know the right answer?
Which type of orbital will be occupied by the electrons of highest energy for the si^4+ ground-state...
Questions


Mathematics, 10.10.2019 20:30

Mathematics, 10.10.2019 20:30

Mathematics, 10.10.2019 20:30



Mathematics, 10.10.2019 20:30


Biology, 10.10.2019 20:30


Chemistry, 10.10.2019 20:30

History, 10.10.2019 20:30

Biology, 10.10.2019 20:30

Social Studies, 10.10.2019 20:30

Biology, 10.10.2019 20:30

English, 10.10.2019 20:30

Mathematics, 10.10.2019 20:30

History, 10.10.2019 20:30

English, 10.10.2019 20:30

Social Studies, 10.10.2019 20:30

![[Ne]3s^{2}3p^{2}](/tpl/images/0460/3364/54c8d.png) .
. 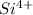 has a +4 charge which means that it has lost 4 electrons. Hence, the electronic configuration of
has a +4 charge which means that it has lost 4 electrons. Hence, the electronic configuration of 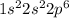 .
. 

