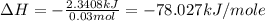Chemistry, 18.01.2020 04:31 Mordred809
2. a neutralization reaction was carried out in a calorimeter. the change in temperature (at) of the solution was 5.6 °c and the mass of the solution was 100.0 g. calculate the amount of heat energy gained by the solution (qsol). use 4.18 j/(g. c) as the specific heat, cs, of the solution.
3. what is the value of qreaction for the neutralization reaction described in number 2?
4. how many moles of phosphoric acid are contained in 50.0 ml of 0.60 m h3po4?
5. what is the value of ahr kj/mol phosphoric acid) if 50.0 ml of 0.60 m reaction in h3po4 was used in the reaction described in number 2?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2
You know the right answer?
2. a neutralization reaction was carried out in a calorimeter. the change in temperature (at) of the...
Questions



Mathematics, 05.01.2022 14:10


Business, 05.01.2022 14:10


Biology, 05.01.2022 14:10

SAT, 05.01.2022 14:10



Computers and Technology, 05.01.2022 14:10




Computers and Technology, 05.01.2022 14:10

Social Studies, 05.01.2022 14:10

English, 05.01.2022 14:20


Mathematics, 05.01.2022 14:20

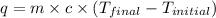
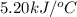
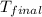 = final temperature
= final temperature 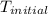 = initial temperature
= initial temperature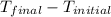 = 5.6°C
= 5.6°C 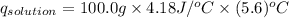
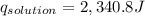
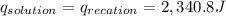
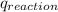 of reaction for the neutralization reaction described in number 2.
of reaction for the neutralization reaction described in number 2.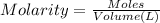

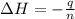
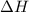 = enthalpy change = ?
= enthalpy change = ?