Chemistry, 18.01.2020 05:31 veronica25681
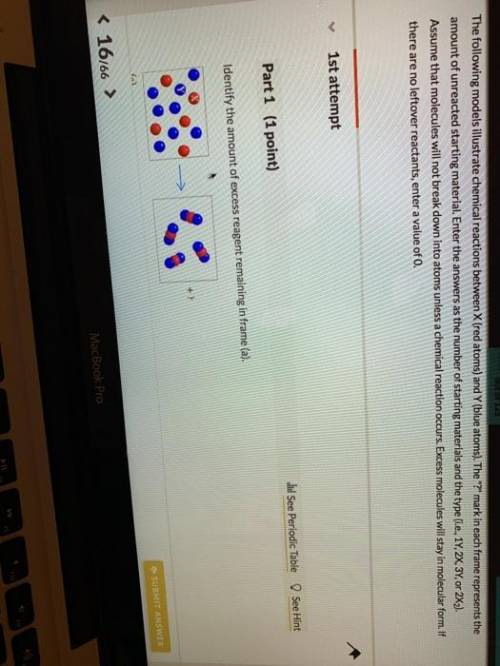
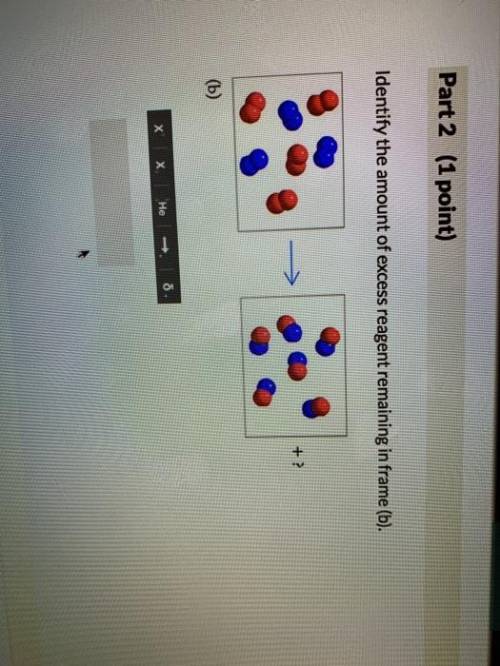
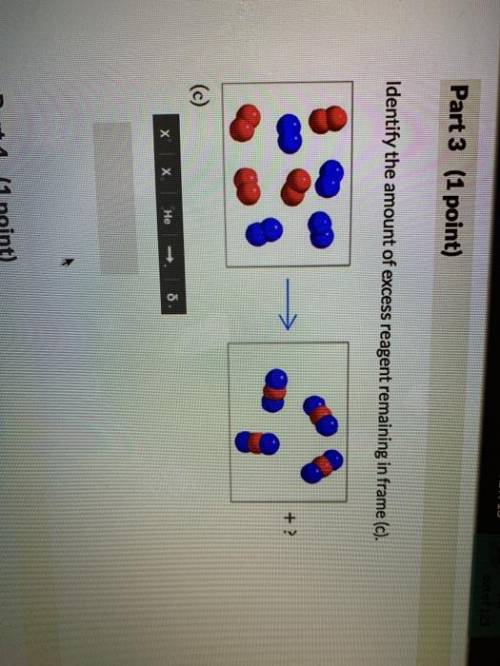
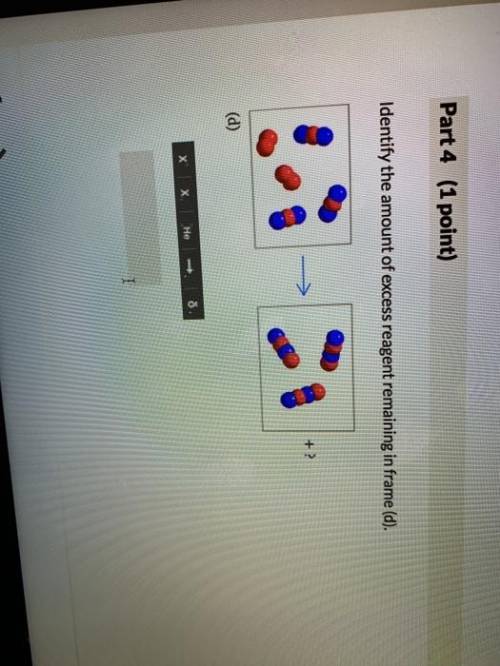
The following models illustrate chemical reactions between x (red atoms) and y (blue atoms). the "? " mark in each frame represents the amount of unreacted starting material. enter the answers as the number of starting materials and the type (i. e., 1y, 2x, 3y, or 2x2). assume that molecules will not break down into atoms unless a chemical reaction occurs. excess molecules will stay in molecular form. if there are no leftover reactants, enter a value of 0.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2
You know the right answer?
The following models illustrate chemical reactions between x (red atoms) and y (blue atoms). the "?...
Questions

Biology, 25.09.2019 08:10



Physics, 25.09.2019 08:10

Social Studies, 25.09.2019 08:10

History, 25.09.2019 08:10


Biology, 25.09.2019 08:10

Computers and Technology, 25.09.2019 08:10




Health, 25.09.2019 08:10

History, 25.09.2019 08:10


Biology, 25.09.2019 08:10

Mathematics, 25.09.2019 08:10

Computers and Technology, 25.09.2019 08:10