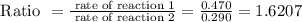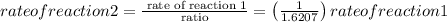Chemistry, 19.01.2020 02:31 amauris77748
Astudent sets up two reactions. reaction 1 uses 0.290 mol/l of reactant, and reaction 2 uses 0.470 mol/l of reactant. how many times faster is reaction 2 compared to reaction 1?
express your answer as a multiple of the rate for reaction 1 to three significant figures. note that reaction 1 is already written for you, so just enter the number.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:20
Which of the following statements is not true? • a. covalent compounds have low melting and boiling points. • ob. covalent bonds between atoms of a compound are relatively weak compared to bonds between molecules. • c. covalent bonds occur between nonmetals. • d. covalent compounds are often gases or liquids.
Answers: 2

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
Astudent sets up two reactions. reaction 1 uses 0.290 mol/l of reactant, and reaction 2 uses 0.470 m...
Questions


Biology, 12.02.2021 14:00

English, 12.02.2021 14:00

Mathematics, 12.02.2021 14:00








Mathematics, 12.02.2021 14:00


Spanish, 12.02.2021 14:00



Mathematics, 12.02.2021 14:00

Advanced Placement (AP), 12.02.2021 14:00

Mathematics, 12.02.2021 14:00

History, 12.02.2021 14:00