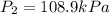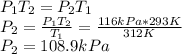The enclosed cabin of a submarine has a volume of 2.4 × 105 liters, a temperature of 312 k, and a pressure of 116 kpa. as people in the cabin breathe, carbon dioxide gas, co2(g), can build up to unsafe levels. air in the cabin becomes unsafe to breathe when the mass of co2(g) in this cabin exceeds 2156 grams. show a numerical setup for calculating the pressure in the submarine cabin if the cabin temperature changes to 293 k

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1
You know the right answer?
The enclosed cabin of a submarine has a volume of 2.4 × 105 liters, a temperature of 312 k, and a pr...
Questions

History, 19.12.2021 01:50

Social Studies, 19.12.2021 01:50


Mathematics, 19.12.2021 01:50

Mathematics, 19.12.2021 01:50

SAT, 19.12.2021 01:50


Mathematics, 19.12.2021 02:00

Business, 19.12.2021 02:00

English, 19.12.2021 02:00







Mathematics, 19.12.2021 02:00


Arts, 19.12.2021 02:00