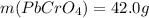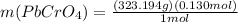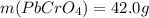Asolution of potassium chromate reacts with a solution of lead(ii) nitrate to produce a yellow precipitate of lead(ii) chromate and a solution of potassium nitrate.
(a) write the balanced chemical equation. (use the lowest possible coefficients. include states-of-matter under the given conditions in your answer.)
(b) starting with 0.130 mol potassium chromate, determine the mass of lead chromate that can be formed.
g


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3


Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1
You know the right answer?
Asolution of potassium chromate reacts with a solution of lead(ii) nitrate to produce a yellow preci...
Questions


Mathematics, 04.03.2021 20:00


Social Studies, 04.03.2021 20:00

English, 04.03.2021 20:00


Mathematics, 04.03.2021 20:00


English, 04.03.2021 20:00


Biology, 04.03.2021 20:00


Mathematics, 04.03.2021 20:00