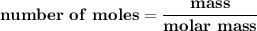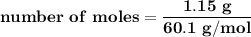Suppose that 1.15 g of rubbing alcohol (c3h8o) evaporates from a 65.0-g aluminum block. if the aluminum block is initially at 25 °c, what is the final temperature of the block after the evapo- ration of the alcohol? assume that the heat required for the vaporization of the alcohol comes only from the aluminum block and that the alcohol vaporizes at 25 °c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2
You know the right answer?
Suppose that 1.15 g of rubbing alcohol (c3h8o) evaporates from a 65.0-g aluminum block. if the alumi...
Questions








Computers and Technology, 12.08.2020 04:01

Physics, 12.08.2020 04:01

Biology, 12.08.2020 04:01


English, 12.08.2020 04:01

Mathematics, 12.08.2020 04:01



Chemistry, 12.08.2020 04:01

English, 12.08.2020 04:01

Physics, 12.08.2020 04:01

Biology, 12.08.2020 04:01