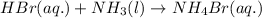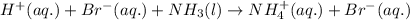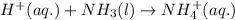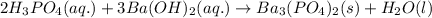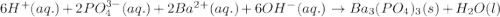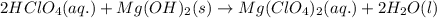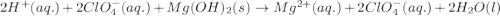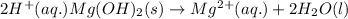Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
Balance the following equations and write the corresponding ionic and net ionic equations:
Questions

Mathematics, 05.02.2022 14:00

English, 05.02.2022 14:00



Mathematics, 05.02.2022 14:00

Mathematics, 05.02.2022 14:00

Mathematics, 05.02.2022 14:00


Mathematics, 05.02.2022 14:00

Mathematics, 05.02.2022 14:00

History, 05.02.2022 14:00

Mathematics, 05.02.2022 14:00

Chemistry, 05.02.2022 14:00

Chemistry, 05.02.2022 14:00



Mathematics, 05.02.2022 14:00

English, 05.02.2022 14:00

Mathematics, 05.02.2022 14:00