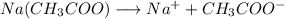Chemistry, 20.01.2020 19:31 officialgraciela67
If you weighed out a small sample of sodium acetate, then dumped it into a beaker of water and stirred it around, which of these statements would not be true? a. the the sodium acetate will fail to dissolve b. the solution will conduct electricity. c. individual sodium and acetate ions are present. d. none of the above

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
You know the right answer?
If you weighed out a small sample of sodium acetate, then dumped it into a beaker of water and stirr...
Questions

Computers and Technology, 07.12.2020 19:50

Mathematics, 07.12.2020 19:50

Mathematics, 07.12.2020 19:50


Social Studies, 07.12.2020 19:50

Mathematics, 07.12.2020 19:50


English, 07.12.2020 19:50




Chemistry, 07.12.2020 19:50

Mathematics, 07.12.2020 19:50

Mathematics, 07.12.2020 19:50

Mathematics, 07.12.2020 19:50

Mathematics, 07.12.2020 19:50

Mathematics, 07.12.2020 19:50