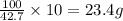Chemistry, 20.01.2020 20:31 harleymichaelbp74jsq
Achemistry student needs of isopropenylbenzene for an experiment. she has available of a w/w solution of isopropenylbenzene in carbon tetrachloride. calculate the mass of solution the student should use. if there's not enough solution, press the "no solution" button.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1
You know the right answer?
Achemistry student needs of isopropenylbenzene for an experiment. she has available of a w/w solutio...
Questions


Mathematics, 08.11.2019 16:31

Mathematics, 08.11.2019 16:31

History, 08.11.2019 16:31

Chemistry, 08.11.2019 16:31

Mathematics, 08.11.2019 16:31

Mathematics, 08.11.2019 16:31




History, 08.11.2019 16:31

Chemistry, 08.11.2019 16:31



Mathematics, 08.11.2019 16:31


Business, 08.11.2019 16:31



English, 08.11.2019 16:31