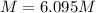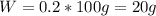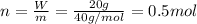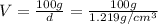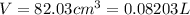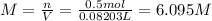Chemistry, 20.01.2020 20:31 madams4450
A20.0%(m/m) solution of naoh (fm 40.00) has a density of 1.219 g/cm'. calculate the solution's molarity. (6.10 m) 2. * 10 w= amountiof solute d- density of solution motanty m= molecular mass ot solute %3d

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1

Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1
You know the right answer?
A20.0%(m/m) solution of naoh (fm 40.00) has a density of 1.219 g/cm'. calculate the solution's molar...
Questions

History, 28.05.2020 07:59

Mathematics, 28.05.2020 07:59

Mathematics, 28.05.2020 07:59

Mathematics, 28.05.2020 07:59


English, 28.05.2020 07:59



Mathematics, 28.05.2020 07:59






History, 28.05.2020 07:59


Mathematics, 28.05.2020 07:59

SAT, 28.05.2020 07:59


Mathematics, 28.05.2020 07:59