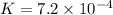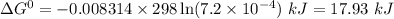Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 23.06.2019 10:30
If a computer chip switches off -on-off in 0.015 us, what is the switching time in nanoseconds?
Answers: 2

Chemistry, 23.06.2019 10:30
If a 20.0ml test tube measures 15.0cm, what is the length in meters?
Answers: 1

Chemistry, 23.06.2019 19:40
Alab technician needs to create 570.0 milliliters of a 2.00 m solution of magnesium chloride (mgcl2). to make this solution, how many grams of magnesium chloride does the technician need? refer to the periodic table for . express your answer to three significant figures.
Answers: 3
You know the right answer?
Calculate δg° for the following reaction from the equilibrium constant at the temperature given. hf(...
Questions

Social Studies, 24.08.2019 19:30

Mathematics, 24.08.2019 19:30


Mathematics, 24.08.2019 19:30

Arts, 24.08.2019 19:30

Social Studies, 24.08.2019 19:30




Mathematics, 24.08.2019 19:30




History, 24.08.2019 19:30


Mathematics, 24.08.2019 19:30

Mathematics, 24.08.2019 19:30

Chemistry, 24.08.2019 19:30

Mathematics, 24.08.2019 19:30

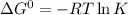
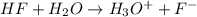
![25^oC=[25+273]K=298K](/tpl/images/0462/9739/df1f6.png)