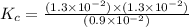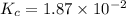Chemistry, 25.12.2019 00:31 valenciafaithtorres
Consider the following reaction: . so2cl2(g)⇌so2(g)+cl2(g) . a reaction mixture is made containing an initial [so2cl2] of 2.2×10−2m . at equilibrium, [cl2]= 1.3×10−2m .. calculate the value of the equilibrium constant (kc).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3
You know the right answer?
Consider the following reaction: . so2cl2(g)⇌so2(g)+cl2(g) . a reaction mixture is made containing...
Questions






Mathematics, 28.07.2021 20:30


Mathematics, 28.07.2021 20:30

Mathematics, 28.07.2021 20:30






French, 28.07.2021 20:40

French, 28.07.2021 20:40


Physics, 28.07.2021 20:40


Mathematics, 28.07.2021 20:40

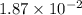
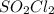 =
= 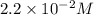
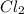 =
= 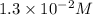
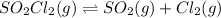

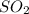 =
= ![K_c=\frac{[SO_2]\times [Cl_2]}{[SO_2Cl_2]}](/tpl/images/0432/3201/33429.png)