
Chemistry, 20.01.2020 21:31 maystrenko53
Ammonia has been studied as an alternative "clean" fuel for internal combustion engines, since its reaction with oxygen produces only nitrogen and water vapor, and in the liquid form it is easily transported. an industrial chemist studying this reaction fills a 500 ml flask with 4.8 atm of ammonia gas and 1.9 atm of oxygen gas at 33 degress celsius. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of nitrogen gas to be 0.63 atm. calculate the pressure equilibrium constant for the combustion of ammonia at the final temperature of the mixture.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

You know the right answer?
Ammonia has been studied as an alternative "clean" fuel for internal combustion engines, since its r...
Questions






Mathematics, 24.07.2019 11:00

Mathematics, 24.07.2019 11:00



Physics, 24.07.2019 11:00

Mathematics, 24.07.2019 11:00



Mathematics, 24.07.2019 11:00




Social Studies, 24.07.2019 11:00

Physics, 24.07.2019 11:00

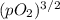 ]
]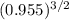 ]
]

