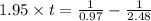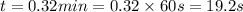The rate constant for the decomposition of nitrogen dioxide no2(g) latex: \longrightarrow⟶ no (g) + 1/2 o2(g) with a laser beam is 1.95 1/mlatex: \cdot⋅ min. find the time, in seconds, needed to decrease 2.48 m of no2 to 0.97 m. hint: what is the order of the reaction? how can you determine that? units of k?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3
You know the right answer?
The rate constant for the decomposition of nitrogen dioxide no2(g) latex: \longrightarrow⟶ no (g) +...
Questions



Mathematics, 07.07.2019 02:20



Mathematics, 07.07.2019 02:20

Mathematics, 07.07.2019 02:20


Arts, 07.07.2019 02:30



Mathematics, 07.07.2019 02:30


English, 07.07.2019 02:30

English, 07.07.2019 02:30


English, 07.07.2019 02:30


History, 07.07.2019 02:30

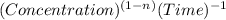
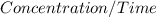 0
0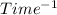 1
1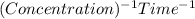 2
2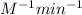 . So, the order of reaction is second order.
. So, the order of reaction is second order.![kt=\frac{1}{[A_t]}-\frac{1}{[A_o]}](/tpl/images/0463/2193/ccade.png)
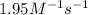
![[A_t]](/tpl/images/0463/2193/5262c.png) = final concentration = 0.97 M
= final concentration = 0.97 M![[A_o]](/tpl/images/0463/2193/dc622.png) = initial concentration = 2.48 M
= initial concentration = 2.48 M