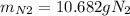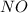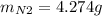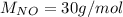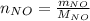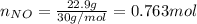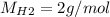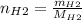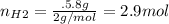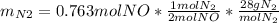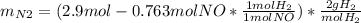Chemistry, 20.01.2020 23:31 jewlbug4358
For the following reaction, 22.9 grams of nitrogen monoxide are allowed to react with 5.80 grams of hydrogen gas. nitrogen monoxide (g) + hydrogen (g)> nitrogen (g) + water (1) what is the maximum amount of nitrogen gas that can be formed? what is the formula for the limiting reagent? grams what amount of the excess reagent remains after the reaction is complete? grams

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3
You know the right answer?
For the following reaction, 22.9 grams of nitrogen monoxide are allowed to react with 5.80 grams of...
Questions

Mathematics, 04.08.2019 03:00

Biology, 04.08.2019 03:10




English, 04.08.2019 03:10

Mathematics, 04.08.2019 03:10

Mathematics, 04.08.2019 03:10



Mathematics, 04.08.2019 03:10

Mathematics, 04.08.2019 03:10

History, 04.08.2019 03:10




English, 04.08.2019 03:10

Mathematics, 04.08.2019 03:10

Mathematics, 04.08.2019 03:10

Mathematics, 04.08.2019 03:10