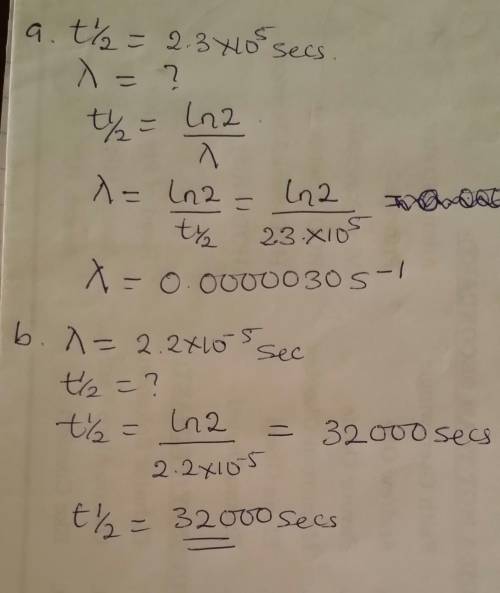Chemistry, 20.01.2020 23:31 ayoismeisjuam
The gas-phase decomposition of so2cl2, so2cl2(g)→so2(g)+cl2(g), is first order in so2cl2. at 600 k the half-life for this process is 2.3×105s.
part a
what is the rate constant at this temperature?
express your answer using two significant figures.
part b
at 320 ∘c the rate constant is 2.2×10−5s−1. what is the half-life at this temperature?
express your answer using two significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1


Chemistry, 23.06.2019 06:30
Which of the following steps is not likely to take place during cellular respiration? (5 points) select one: a. oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. c. simple sugar breaks down. d. energy is used up.
Answers: 1
You know the right answer?
The gas-phase decomposition of so2cl2, so2cl2(g)→so2(g)+cl2(g), is first order in so2cl2. at 600 k t...
Questions

English, 09.02.2021 19:40

Mathematics, 09.02.2021 19:40

Mathematics, 09.02.2021 19:40

Arts, 09.02.2021 19:40


Mathematics, 09.02.2021 19:40


Mathematics, 09.02.2021 19:40


Business, 09.02.2021 19:40

Mathematics, 09.02.2021 19:40

Mathematics, 09.02.2021 19:40

History, 09.02.2021 19:40


Mathematics, 09.02.2021 19:40

Computers and Technology, 09.02.2021 19:40

Mathematics, 09.02.2021 19:40

Mathematics, 09.02.2021 19:40

Mathematics, 09.02.2021 19:40