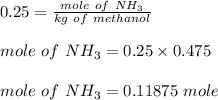Chemistry, 21.01.2020 01:31 jaylafennell101
What mass (in g) of nh3 must be dissolved in 475 g of methanol to make a 0.250 m solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Chemistry, 23.06.2019 05:30
Find the midpoint of a segment with endpoints of 4-3i and -2+7i
Answers: 2

Chemistry, 23.06.2019 10:30
Describe the hybridization of each carbon and nitrogen atom in each of the following structures
Answers: 1
You know the right answer?
What mass (in g) of nh3 must be dissolved in 475 g of methanol to make a 0.250 m solution?...
Questions

Social Studies, 30.06.2019 00:00

Mathematics, 30.06.2019 00:00

History, 30.06.2019 00:00

Mathematics, 30.06.2019 00:00

History, 30.06.2019 00:00




Mathematics, 30.06.2019 00:00



English, 30.06.2019 00:00


Mathematics, 30.06.2019 00:00

Mathematics, 30.06.2019 00:00

English, 30.06.2019 00:00


Physics, 30.06.2019 00:00

Mathematics, 30.06.2019 00:00