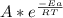Chemistry, 21.01.2020 01:31 cierramcdonald
A)if it takes 0.20 min to decompose 15% of a 0.300 m solution of nitrosyl chloride, what is k(the rate constant)? the decomposition of nitrosyl chloride is a second order reaction: nocl(> no(g)+1/2cl2(g)b)calculate the activation energy? when the temperature increases from 15c to 25c for a certain reaction, its rate constant doubles. calculate the activation energy, ea+ (remember: with r=8.31 j/mol. k, ea must be expressed in j/mol)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Two nitro no2 groups are chemically bonded to a patch of surface. they can't move to another location on the surface, but they can rotate (see sketch at right). it turns out that the amount of rotational kinetic energy each no2 group can have is required to be a multiple of ε, where =ε×1.010−24 j. in other words, each no2 group could have ε of rotational kinetic energy, or 2ε, or 3ε, and so forth — but it cannot have just any old amount of rotational kinetic energy. suppose the total rotational kinetic energy in this system is initially known to be 32ε. then, some heat is removed from the system, and the total rotational kinetic energy falls to 18ε. calculate the change in entropy. round your answer to 3 significant digits, and be sure it has the correct unit symbol.
Answers: 2

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3
You know the right answer?
A)if it takes 0.20 min to decompose 15% of a 0.300 m solution of nitrosyl chloride, what is k(the ra...
Questions

History, 05.09.2019 19:20



Biology, 05.09.2019 19:20




English, 05.09.2019 19:20

History, 05.09.2019 19:20

English, 05.09.2019 19:20



History, 05.09.2019 19:20

Mathematics, 05.09.2019 19:20

Mathematics, 05.09.2019 19:20




Mathematics, 05.09.2019 19:20

Mathematics, 05.09.2019 19:20