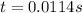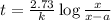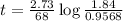At 1000°c, cyclobutane (c4h8) decomposes in a first-order reaction, with the very high rate constant of 68 1/s, to two molecules of ethylene (c2h4). the initial cyclobutane concentration is 1.84. how long will it take for 52% of the cyclobutane to decompose? enter to 4 decimal places.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1
You know the right answer?
At 1000°c, cyclobutane (c4h8) decomposes in a first-order reaction, with the very high rate constant...
Questions


Advanced Placement (AP), 19.01.2021 04:20


Mathematics, 19.01.2021 04:20





Mathematics, 19.01.2021 04:20



Mathematics, 19.01.2021 04:20

Mathematics, 19.01.2021 04:20

Mathematics, 19.01.2021 04:20

Mathematics, 19.01.2021 04:20


Mathematics, 19.01.2021 04:20

Biology, 19.01.2021 04:20


History, 19.01.2021 04:20