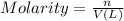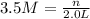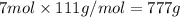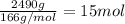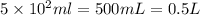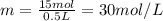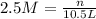Chemistry, 09.10.2019 21:40 arthurcasella
1. how many grams of cacl2 would be required to produce a 3.5 m (molar) solution with a volume of 2.0 l? . 2. what is the molarity of a 5.00 x 102 ml solution containing 2490 g of ki? . 3. how many moles of lif would be required to produce a 2.5 m solution with a volume of 10.5 l? .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

You know the right answer?
1. how many grams of cacl2 would be required to produce a 3.5 m (molar) solution with a volume of 2....
Questions



Mathematics, 21.05.2021 08:40


Biology, 21.05.2021 08:40



Chemistry, 21.05.2021 08:40

English, 21.05.2021 08:40

Chemistry, 21.05.2021 08:40

Mathematics, 21.05.2021 08:40

Mathematics, 21.05.2021 08:40

Chemistry, 21.05.2021 08:40



Social Studies, 21.05.2021 08:40



Advanced Placement (AP), 21.05.2021 08:50