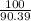Chemistry, 21.01.2020 05:31 fangkronos
Suppose you have a 100-gram sample of each of the following compounds. which sample contains the largest number of moles of compound? chromium(iii) chlorideammoniamagnesium chloridephosphoric acidpotassium chloridenitrogen chlorate

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1
You know the right answer?
Suppose you have a 100-gram sample of each of the following compounds. which sample contains the lar...
Questions

Biology, 18.11.2019 23:31

Biology, 18.11.2019 23:31






Mathematics, 18.11.2019 23:31








Mathematics, 18.11.2019 23:31

English, 18.11.2019 23:31



Mathematics, 18.11.2019 23:31

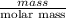
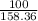
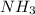 =
= 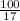
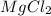 =
= 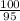
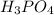 =
= 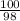
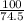
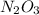 =
= 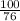
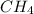 =
= 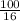
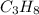 =
= 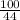
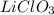 =
=